The Atom
Objectives:† Review the structure of the atom and its constituents.
In chemistry, the atom is the fundamental building block of chemical structures.† Unique combinations of atoms lead to the diverse array of molecules that form the universe.† Democritus, around 400 BC, first proposed the idea of an unchangeable atom.† However, the Greeks had no experiments to test this idea.† Around 1800, John Dalton performed many experiments to measure the ratios of the masses of elements in compounds.† From the results of these experiments, he hypothesized that matter consists of atoms and in 1808 he published the Atomic Theory of Matter.† Though his points are not entirely true, it is very important to understand them and appreciate the foresight of Dalton.† Remember there was no way that Dalton could see the atom at the time of his publishing.† Today, however, modern instruments provide direct evidence that atoms exist.
Dalton's
Atomic Theory of Matter
- Elements and compounds consist of indivisible atoms.
- Atoms of a given element are identical (meaning they have the same mass and properties).
- Atoms retain their identity in all chemical reactions.† This is basically the Law of Conservation of matter stating that an equal amount of mass exists before and after a reaction.
- Atoms combine in fixed ratios of small integers to form compounds.† This is close to the Law of Definite Proportions.
The atom continues to be fundamental in the field of chemistry as well as other sciences; however, today we understand the atom in more depth than in Daltonís time.
The
General Structure of the Atom
The atom consists of protons and neutrons in a central nucleus with electrons distributed outside in an electron cloud or orbital.† (However, as you will learn later, determining where exactly these electrons are is very difficult to say the least).
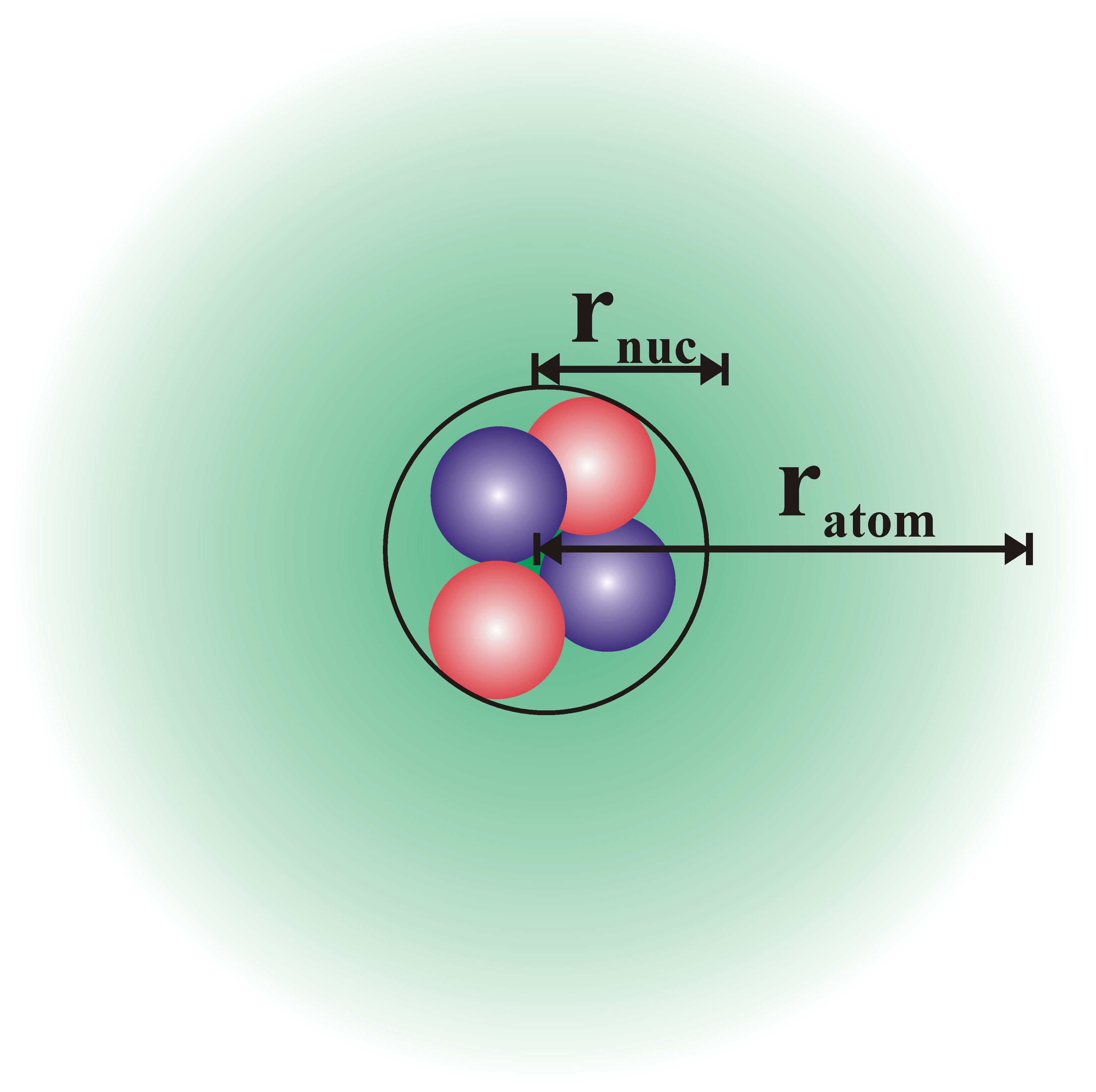
This Helium atom contains 2 protons and 2 neutrons within the nucleus.† The 2 electrons (in a neutral atom) are outside of the nucleus in the electron cloud.† The protons have a positive charge, the neutrons are neutral and the electrons possess a negative charge.† The cartoon to the right is not drawn to scale-- the radius of the atom is much greater than the radius of the nucleus.† In fact ratom>104 rnuc.† What does this mean? †Answer
Masses of
atomic components:
p+ = 1.67262 x 10-27 kg
n = 1.67493 x 10-27 kg
e- = 9.1094 x 10-31 kg
What does this tell you about the atom? †Answer
Atomic
Symbols
Each element is named by a symbol.† When writing elements we usually include the mass number and occasionally the atomic number. If we continue with the example above, He is represented by:
![]()
4 is the mass number (A), which is the number of protons plus the number of neutrons.
2 is the atomic number (Z), which is the number of protons. The atomic number often is not included because the element name also tells the number of protons.† If the number of protons changes, then it becomes a different element.† For example Helium will always have 2 protons; if you add a proton, it then becomes Li.† (By the way, this is a nuclear process and is not easy to accomplish!)
In a neutral atom, the number of electrons must equal the number of protons.
However, once a charge is added the number of electrons does not equal Z.
![]() ††† # of e = Z††††††† Neutral He atom
††† # of e = Z††††††† Neutral He atom
![]() † # of e < Z††††††† He cation
† # of e < Z††††††† He cation
![]() † # of e > Z††††††† He anion
† # of e > Z††††††† He anion
Note that the number of protons and neutrons are not changing.† It is only the number of electrons that change.
Cation: a positively charged ion.
Anion: a negatively charged ion.
Problems:
1.†† How many protons, neutrons and electrons are there in each of the following elements?
††††† a.†† ![]() †† ††††††††††† Answer
†† ††††††††††† Answer
††††† b.†† ![]() ††††††††† Answer
††††††††† Answer
††††† c.†† ![]() †††††††††† Answer
†††††††††† Answer
††††† d.†† ![]() †††††††††† Answer
†††††††††† Answer
2.†† Complete the following chart:
|
Element Symbol |
# of protons |
# of neutrons |
# of electrons |
net charge |
|
|
|
|
|
|
|
|
15 |
15 |
15 |
|
|
|
33 |
42 |
|
-3 |
|
|
54 |
77 |
|
0 |
|
|
|
58 |
43 |
+2 |
Summary:† Now you should feel more comfortable with the structure and parts of the atom. Also you should be able to write out atomic symbols.
Questions:† Are you satisfied with the picture of the atom, or in other words, do you think that is what the atom really looks like?
Why is it difficult to determine where the electrons are?
Are the electrons just scattered around the nucleus or do you think there is an order?† And what does an ďelectron cloudĒ mean?
We said that Daltonís Atomic Theory is not entirely true.† Under what circumstances are they not true?† Hint: each point has an exception.
Donít worry if you donít know all of these answers yet, you will learn about this in Chemistry 111 and 112.† But you should be able to answer the last question certainly by the end of this tutorial.