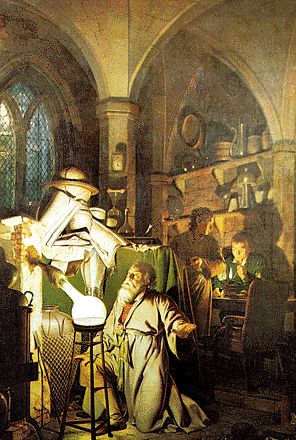The Two-Column Method: Organizing an Attack on Chemistry
Word-Problems
It is important to realize that not everyone thinks about or solves problems
in the same way. Some people can read a problem and very quickly they have an
idea or feeling about how they will reach their solution. Sometimes the most
direct route to an answer is not so obvious and a little advanced planning makes
things run more smoothly. In this example, we demonstrate the working of a problem
via the two-column method, which is really just a way of organizing a plan of
attack.
The problem is fairly complex and requires a number of logical and mathematical
steps for its solution. We will walk you through the problem, along the way
demonstrating how you might organize your approach using this two-column method.
Of course, the first step is to read the problem, so here it is:
Question
- In the painting "The Discovery of Phosphorus" (shown) by the English painter Joseph Wright (1734-1797) an alchemist is depicted heating urine paste in a reaction vessel over coals. The brilliant light due to the reaction of phosphorus with oxygen is one of the products of the chemical reaction:
P4 (s) + n O2 (g)  PxOy (s) + light + heat.
PxOy (s) + light + heat.
The resulting solid phosphorus oxide PxOy can then be treated with water to produce phosphoric acid according to the reaction:
PxOy (s) + 6 H2O (l)  4 H3PO4 (aq).
4 H3PO4 (aq).
You attempt to duplicate the first of these chemical reactions in a closed system. Your apparatus consists of a flask connected via tubing to a cylinder (with a frictionless, moveable piston-head seal) containing oxygen gas. The oxygen gas cylinder has graduated markings so you can observe the volume of oxygen gas inside of the cylinder. As oxygen is consumed in the reaction the piston head moves. For each mole of electrons removed from phosphorus, what change in volume do you observe in the oxygen cylinder? The density of oxygen gas at room temperature is 1.308 g/L. Assume that the gas cylinder is thermally insulated from the reaction vessel so the oxygen gas in the cylinder remains at room temperature.
General Comments
Are you asking yourself, "How do I begin?" Perhaps it is better to start by asking
yourself, "Where am I going?" and to follow this up by asking yourself, "How do
I get there?" We know where we must end up. We have to find the volume of O2
(g) produced for each mole of electrons removed from phosphorus. We have the
density of O2 (g) and some chemical equations with some missing constants
x,y and n (i.e., unbalanced equations). The wording tips us off to the fact that
at least one of these reactions is a redox reaction with the result that phosphorus
is oxidized.
Solution Outline
Concepts Involved
First it may be a good idea to make a list of the concepts dealt with in the problem. You could either do this in your head or on paper.
- Balancing Chemical Equations
- Redox Reactions
- Unit analysis (moles to liters, mole ratios, etc.)
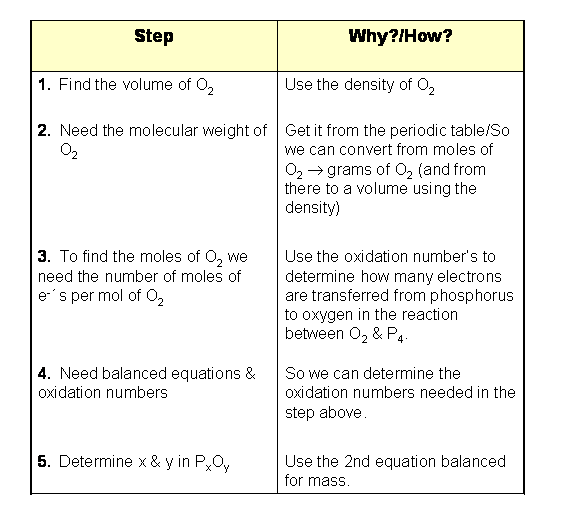
The Outline of Steps
Start by listing the final desired quantity and how to get/why you want that
quantity (in two separate columns). Then, in reverse order, list the steps you
have to perform in order to get there. The last item in your list is your starting
point. Essentially, we are making a reverse-outline of how we will solve the
problem.
The Worked Solution
By balancing the second equation, it is determined that x = 4, y = 10:
P4O10 (s) + 6 H2O (l)  4 H3PO4 (aq). [Note that there is no change in the oxidation
state of phosphorus in this equation.]
4 H3PO4 (aq). [Note that there is no change in the oxidation
state of phosphorus in this equation.]
With this information in hand, we can balance the first chemical equation and determine the number of moles of O2 consumed
per mole of P4 reacted. We can also determine the oxidation states of all the elements in the balanced reaction.
P4 (s) + 5 O2 (g)  P4O10 (s) + light + heat.
P4O10 (s) + light + heat.
The oxidation numbers are P(0) in P4; O(0) in O2; P(+5),
O(-2) in P4O10. Using the oxidation numbers and the stoichiometry in
the balanced equation we know that there are 20 moles of electrons transferred to phosphorus atoms for
every 5 moles of O2 consumed. Now we have everything we need to execute the calculation and answer the question.

Return to the Hints Page